Inject, Cool Tech and Keto Effect

All Science News articles summarize a research study and are not an official opinion, endorsement or position of the Parkinson’s Foundation.
Every year, the Movement Disorder Society (MDS) hosts an international congress where the top minds in the field share ideas and the latest in research. The 2018 congress took place this October in Hong Kong, China, where we found three particularly promising new Parkinson’s disease (PD) studies that not only provide hope and direction for the future, but also a new avenue you may want to explore with your healthcare provider, right now.
Inject
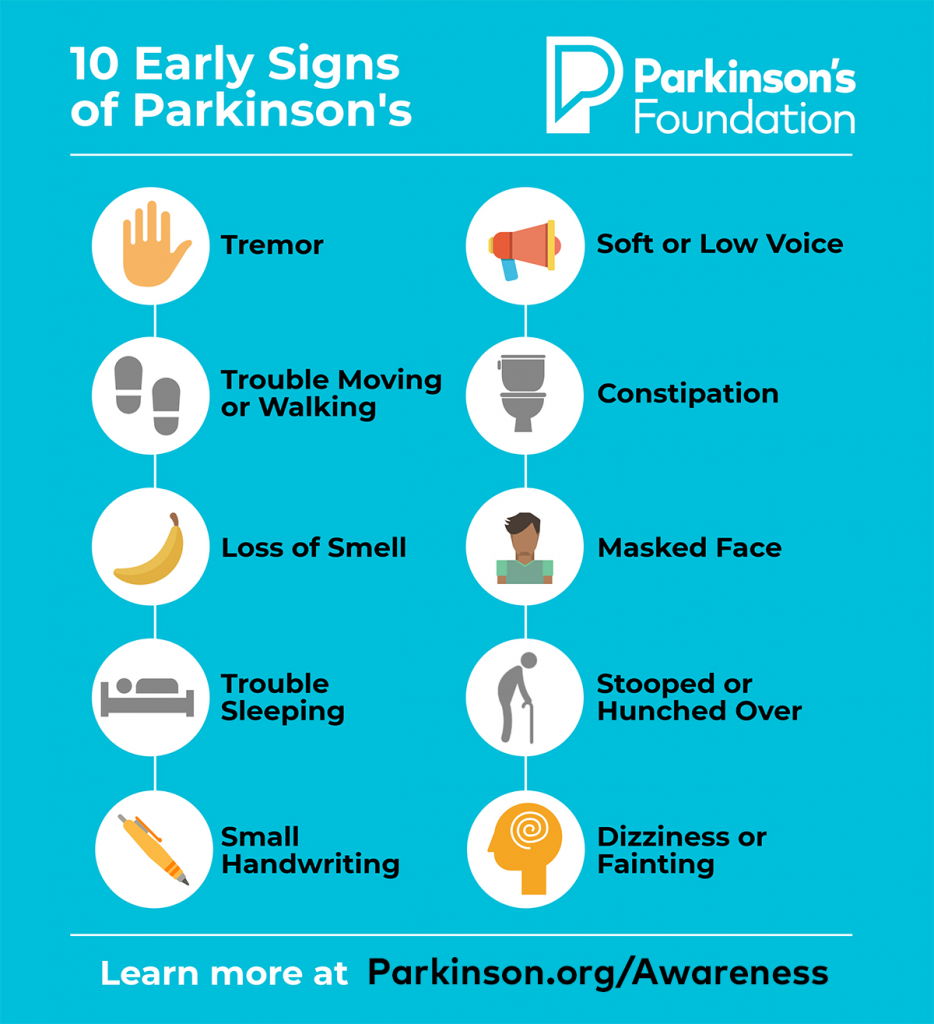
People tend to think of Parkinson’s as an overall body movement disorder. However, it’s not uncommon for PD to negatively impact the facial muscles, mouth, throat and even vocal cords — which can make it difficult to chew, eat, swallow, and speak with audible volume. A medical procedure where a collagen gel is injected directly into the vocal chords may help. Called vocal fold augmentation, the gel filler (specifically, carboxymethylcellulose) provides a thicker, more resilient cushion for the vocal cords. This is not a new procedure being tested, it’s a treatment that has been used for many years to treat vocal cord atrophy, just not in people with PD.
Inspired by a patient who told his otolaryngologist that he wished something could just be injected in his throat, a clinical trial began using the injected gel in 29 people with PD. Researchers measured results in: overall severity, roughness, breathiness, strain, pitch and loudness. They also measured glottis closure timing (essential in airway protection, so food won’t go down the wrong pipe), and supraglottic constrictions, which involves throat constriction above the vocal chords.
In the one-month follow-up, statistically significant improvements were achieved in overall severity, breathiness, loudness and glottic closure time. People’s eating and swallowing ability remained the same. According to the study authors, the positive effects of the gel will wear off in about three months; thereby, providing proof that the injection worked. If that is indeed the case, their next step involves injecting a calcium hydroxyapatite paste, which the researchers suggest could last up to 18 months – at which time, patients may receive another injection.
Learn More
The Parkinson’s Foundation believes in empowering the Parkinson’s community through education. Learn more about speech, swallowing now or by calling our free Helpline at 1-800-4PD-INFO (473-4636).
Cool Tech
Innovations in personal, portable technology to improve Parkinson’s care, health outcomes and overall management for diseases such as diabetes, epilepsy and asthma, have become a major focus of research. And now, researchers may have identified a technology to help people with certain aspects of PD. Called a Parkinson's Kinetigraph (PKG), this wearable device contains an accelerometer that measures and records information on motor patterns, impulsiveness, periods of sleep and medication response. In a recent study involving 70 people with PD, doctors were asked to provide what their management plan for each person would be, before and after receiving the PKG results.
The clinical findings and the PKG were essentially the same for 80% of participants. However, utilizing the additional PKG data resulted in 24 of the PD participants (34%) receiving changes in their clinical management, including altering their medication dosing, as well as recommending advanced therapies such as apomorphine or deep brain stimulation. For two participants, based on their poor response to therapy recorded by the PKG, their Parkinson's diagnosis was questioned. As to whether or not these PKG-inspired clinical management changes will lead to longer-term improvements in people’s health outcomes and/or quality of life has yet to be determined. However, if it does, this technology could be life-changing.
Learn More
Learn more about wearable technology by visiting Assistive Technology & Devices.
Keto Effect
We all know that following a healthy diet is essential for overall health. But which diet is best for people with Parkinson’s? A recent pilot study sought to compare a low fat, high carbohydrate diet (which is thought to increase dopamine levels in the brain), with the high fat, low carbohydrate ketogenic diet (or ‘keto’ as it’s usually referred) — which may help with diminished mitochondrial energy metabolism. A total of 47 study participants with PD were randomly assigned to follow one of the two diets over an eight-week period. The total calories were identical, as was the total protein content — because protein interferes with levodopa absorption.
Overall, following both diets resulted in improvements in movement and non-movement symptoms. However, the group following the keto diet experienced a greater improvement in non-motor symptoms, as compared to the low fat/high carb diet (41% vs 11%, respectively), including experiencing less urinary problems, pain, fatigue, daytime sleepiness and cognitive impairment. This is particularly significant because non-motor symptoms are less responsive to the medication levodopa. In terms of adverse effects, the most common for those following the low fat/high carb diet was being hungry; and for the keto diet, some experienced a transient increase in PD tremor/rigidity. Both groups also lost weight. Bear in mind that this trial was only 2-months long and there was also no control, or normal diet for comparison. Nonetheless, these study findings suggesting the keto diet may be useful for non-motor symptoms are encouraging.
Learn More
The Parkinson’s Foundation believes in empowering the Parkinson’s community through education. Learn more by reading Diet & Nutrition or listening to our podcast episode: The Importance of Good Nutrition for People with Parkinson’s.
Have specific questions about nutrition and diet? Call our free Helpline at 1-800-4PD-INFO (473-4636).
Related Blog Posts

20 Parkinson’s-Friendly Gifts

8 Tips for Traveling with Parkinson’s