Celebrating 12 Milestones that Defined 2025
🧠 What will you learn in this article?
This article highlights the ways the Parkinson’s Foundation helped people living with Parkinson’s and the Parkinson’s community in 2025. It highlights:
- How we advanced research through funding grants and evolving our genetics study.
- Funded local community programs.
- Launched new policy effort to improve care and research through advocacy.
- Spread Parkinson’s awareness through programs, campaigns and resources.
2025 was a remarkable year for the Parkinson’s Foundation. Over the course of 365 days, we advanced Parkinson’s disease (PD) research while working hard to represent the one million people in the U.S. living with this neurodegenerative disease. We strengthened our connections among care partners and everyone serving the PD community.
With your support, we launched new initiatives and vital PD resources, stayed fast in our commitment to improving PD care and research, and empowering the community through education and new resources.
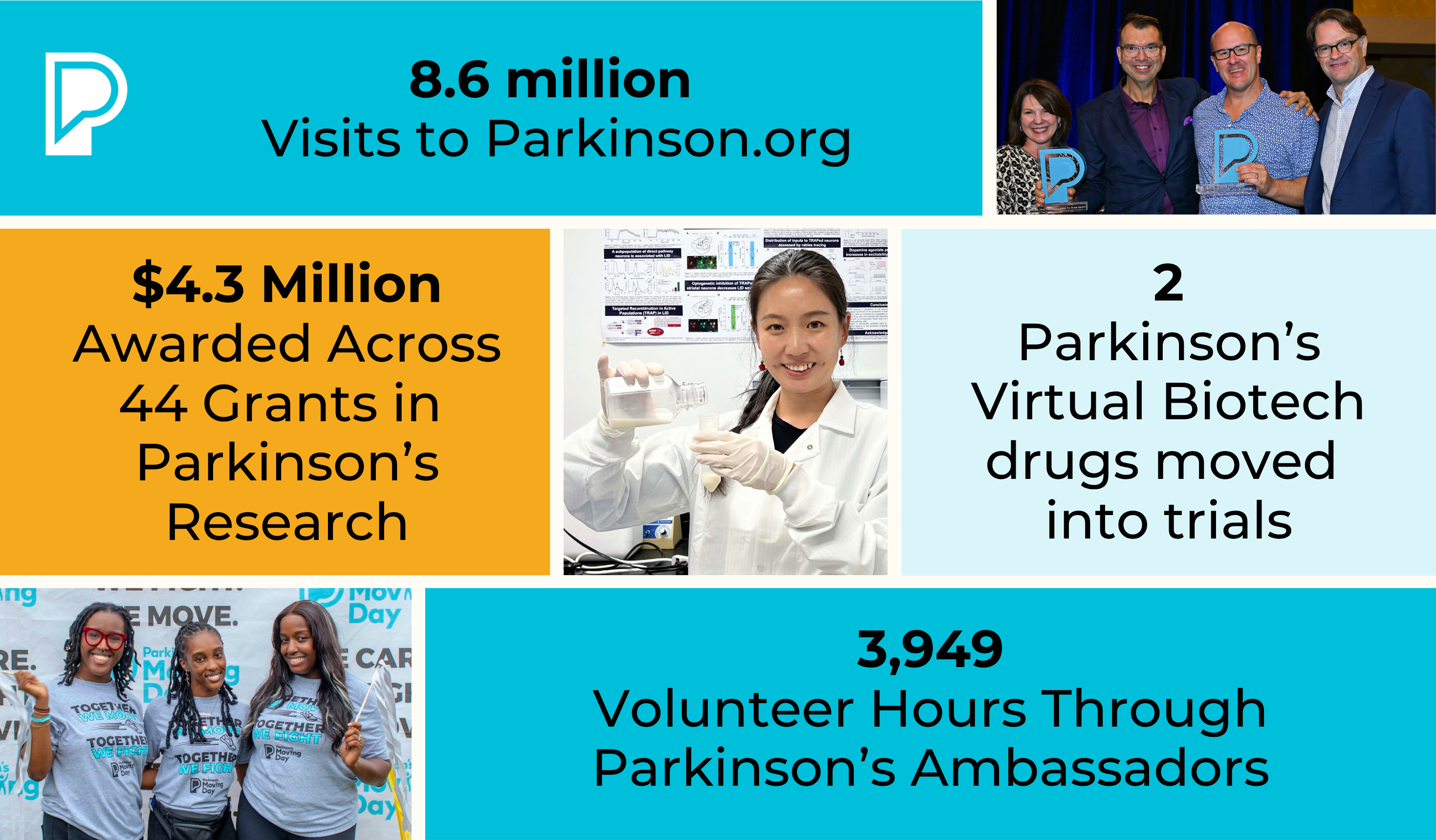
Thanks to YOU, here are the top 12 ways we made a difference this year:
1. Awarded more than $4.3 million in high-risk, high-reward research.
In a year when federal funding for disease research sharply declined, we significantly increased our investment in Parkinson’s research. We awarded more than $4.3 million across 44 grants. We are proud to fund scientists pursuing innovative studies across diverse areas of PD — driving the development of new therapies, treatments and ultimately a cure for the 10 million people worldwide living with this neurological disease.
Meet Jeff Kim, PhD
2025 Parkinson’s Foundation Postdoctural Fellow
Dr. Kim is leveraging AI (artificial intelligence) to advance genetics research. His research seeks to understand how overlapping PD mutations may influence the risk of developing PD.

2. Reached 30,000+ participants in our genetics study.

In 2025, PD GENEration: Powered by the Parkinson’s Foundation reached unprecedented numbers including:
-
Providing genetic testing and counseling to 30,000+ people with Parkinson’s, at no cost.
-
Finding that approximately 12-13% of participants carry a genetic link to PD.
-
Expanded study to a total of 77 testing sites worldwide and counting — adding sites in Mexico, Colombia, Chile, Peru and El Salvador.
We also launched an exciting new pilot program, PD Trial Navigator, to help advance PD GENEration’s goal of accelerating genetic-focused clinical trials. This program helps inform PD GENEration participants about Parkinson’s genetic trials they may qualify for based on their genetic results.
3. Launched new policy effort to accelerate PD treatments and care.

In 2025, we launched new policy initiatives aiming to empower the PD community through advocacy. Highlights include:
-
Appointing Andi Lipstein Fristedt as our first Executive Vice President and Chief Strategy and Policy Officer to guide our policy efforts at the federal, state and local levels.
-
Hosting a national roundtable on Parkinson’s care and innovation to identify national priorities to improve care for people with PD.
-
Co-hosted the 2025 Parkinson's Policy Forum, where 250 people with PD, family members and advocates from 45 states gathered in Washington, D.C. to urge Congress to accelerate progress toward better treatments and a cure.
Sign up for our emails to keep up to date with advocacy efforts
4. Funded local Parkinson’s programs in 38 states.

We awarded more than $1 million in community grants for programs that help people living with PD across 38 states. Our 2025 grants fund local programs that provide exercise and educational support for people with PD and their care partners and address mental health needs. Since 2011, the Foundation has devoted more than $12.7 million in community-based programs, reaching a combined 81,000 people with PD and care partners.
Pictured: Parkinson’s Foundation Community Grantee, Parkinson's Exercise Program For You, in Dana Point, CA, offers PD-tailored exercise programs.
To find your nearest exercise or wellness class, visit your local chapter’s webpage or call our Helpline at 1-800-4PD-INFO (1-800-473-4636).
5. Appointed our first-ever Chief Medical Officer.

This year, we welcomed Sneha Mantri, MD, MS, as Chief Medical Officer of the Parkinson’s Foundation. A nationally recognized movement disorders specialist and educator, Dr. Mantri believes in getting to know her patients and personalizing their treatments. “I'm excited to bring that philosophy of care to this role and address the needs of people with Parkinson’s on a national scale,” she said.
Look out for virtual events featuring Dr. Mantri in 2026.
Learn more about Dr. Mantri here
6. Moved two Parkinson’s Virtual Biotech drugs into trials.
Parkinson’s Virtual Biotech is a research-driven investment fund we support alongside Parkinson’s UK. In 2025 we shared two exciting advances:
-
Project ASPro-PD became the first Parkinson’s Virtual Biotech project to enter a large phase 3 trial, assessing whether ambroxol (a common cough medicine ingredient) can slow the progression of Parkinson’s. This trial is the closest to delivering a new treatment.
-
A new drug from NRG Therapeutics, designed to repair the mitochondria that power brain cells, is advancing to clinical trials for Parkinson’s and ALS (amyotrophic lateral sclerosis). This progress was made possible through early investment from the Parkinson’s Virtual Biotech, proving how our venture philanthropy model fuels innovation — turning bold ideas into real possibilities for people living with Parkinson’s and making investments less risky for future funders.
Learn more about the Parkinson’s Virtual Biotech
7. Launched new resources to help people optimize their PD care.
We know that healthcare appointments for Parkinson’s can feel overwhelming. Which is why we published new content and tips dedicated to help people with PD and care partners advocate for their best care. Use our Steps to Prepare for a Parkinson’s Appointment worksheet for a step-by-step guide to choosing your top three appointment topics.
Learn how to optimize your Parkinson’s care
8. Raised $263,000 on Parkinson’s Foundation Day of Giving.

Our incredible community came together and made our third annual Day of Giving the most successful so far, raising double the amount raised in 2024. Our steadfast supporters made this special day a success, raising awareness and funds to support our mission to make life better for people with Parkinson’s disease.
9. Facilitated 3,949 community service hours through Parkinson’s Ambassadors.

Volunteers are essential to our mission and help us localize our reach. This year, we trained 239 new Parkinson’s Foundation Ambassadors and brought all our volunteers together at our national Volunteer Leadership Summit.
Etana Soloman joined our People with Parkinson’s Advisory Council to add her voice and help represent young caregivers and people like her mother who are in the later stages of PD. “Being able to care for my mom is truly an honor” Read her story.
Find a volunteer opportunity near you
10. Reached 8.6 million visits to Parkinson.org and expanded Spanish-language engagement.
Parkinson.org reached a record of 8.6 million visits, including 1.3 million visits to our Spanish content. Every page visit represents an opportunity to connect people with life-changing resources, digital events and actionable ways to help make life better for people with Parkinson’s.
Hispanic and Latino members of the PD community face distinct barriers to living well with Parkinson’s. In 2025, we published new Spanish pages on dementia, caregiving, vertigo, depression, hospital safety and more (explore these pages in English, too: dementia, caregiving, vertigo, depression, hospital safety).
11. 20,000 participants raised more than $8.3 million through community fundraising events.

Parkinson’s Foundation community fundraisers raised an impressive $8.3 million to advance PD research, access to care and life-changing resources in 2025. Together, every person who participates in Moving Day, A Walk for Parkinson’s, Parkinson’s Champion and Parkinson’s Revolution bring us closer to a cure.
Two years after his diagnosis, Brooke Ramsey found Moving Day Columbus. For the last 14 years his family has raised more than $117,000 to help make life better for people with Parkinson’s. Read his story.
Become a Parkinson’s Champions
Join us for Parkinson’s Revolution
12. Engaged with our audience through two awareness campaigns.
In April, we introduced the world to PAM, your guide to Parkinson’s Awareness Month. To raise PD awareness, PAM shared essential information, tips and resources about PD on our social media channels and website.

In April we:
- Posted 5 new videos highlighting PD facts everyone should know.
- Reached 2+ million visits to Parkinson.org — our most page views in a single month!
- Earned 914,000 impressions across our social media posts
Follow us on social media to help spread Parkinson’s awareness
In November, for National Family Caregivers Month, we amplified the diverse experiences of caregiving through our Real Care. Anywhere. campaign. We provided tailored resources for three types of caregivers including those caring for someone living with Parkinson’s, those providing care from a distance and those managing PD alone.
Explore our care partner resources
We are setting bold goals for 2026 to create an even greater impact on the Parkinson’s community — and your support makes it possible.
Related Materials
Related Blog Posts

10 Tips for Playing Pickleball to Stay Active with Parkinson’s