Ending Parkinson's Disease

Parkinson’s disease (PD) is now the world’s fastest growing brain disorder, even faster than Alzheimer’s. Ten million people live with Parkinson's world-wide. Over the past 25 years, the number of people with Parkinson’s has more than doubled. At this current rate, the number will double again in the coming generation. In the U.S., the number of Americans with the condition has increased by 35% in the last 10 years alone. We must act to stop Parkinson’s disease.
Four authors (three PD specialists and a neuroscientist) wrote a book called Ending Parkinson’s Disease that highlights the rise of the disease, the factors contributing to the increase and what steps we can take together to help end the disease.
Our prescription for action includes a “PACT” that details steps we can do to:
1. Prevent the disease
Several environmental factors, including air pollution, heavy metals, certain pesticides, and industrial chemicals like trichloroethylene are linked to PD. One pesticide called paraquat more than doubles the risk of developing Parkinson’s disease, kills the weeds that RoundUp cannot and has been banned by 32 countries, including China. Yet, use in the U.S. has doubled in the past decade. Despite a petition signed by more than 100,000 members of the Parkinson’s community, the U.S. Environmental Protection Agency has failed to act. We need to ban this pesticide and take other actions to minimize the risk of Parkinson’s disease from the foods we eat, the water we drink and the air we breathe.
2. Advocate for better policies and resources
In addition to better environmental policies, to end Parkinson’s disease will require additional resources. The National Institute of Health (NIH) funds $3 billion per year to enhance our understanding of HIV. This research has likely prevented millions, including many of us from ever developing the disease and led to numerous treatments that now makes a HIV a chronic, rather than rapidly fatal condition. By contrast the NIH funds less than $200 million per year for Parkinson’s disease. We need to change that.
3. Care for all affected
Many individuals with Parkinson’s do not see a neurologist or specialist for PD. Those who do not are more likely to fracture their hip or be placed in a skilled nursing facility. Expert care models, like ParkinsonNet developed by Bas Bloem, MD, and his colleagues, telemedicine and Centers of Excellence can provide better care to almost anyone anywhere. We need to embrace such models and ensure that Medicare, supported by taxpayers like you, does the same.
4. Treat the disease with novel therapies
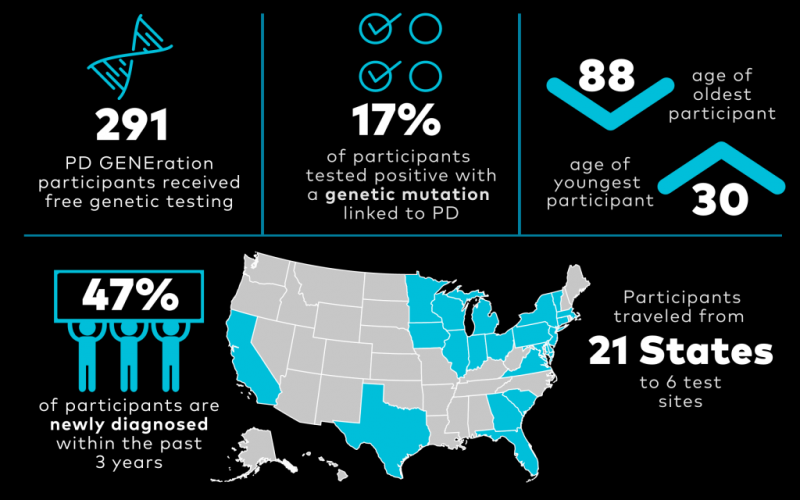
The most effective medication for Parkinson’s remains a 50-year-old drug, levodopa. While effective, it does not address the underlying disease and has its own complications. To develop a new generation of therapies aimed at the underlying pathology of the disease, we need better, objective measures of the disease and to support efforts to develop gene-targeted therapies. The PD GENEration study and other efforts can help individuals better understand their genetic risk of the disease. Additional surgical interventions can also advance our treatment of those already affected by the disease.

All of these will require the collective action of all us. The same collective action that changed the course of polio through a March of Dimes and the course of HIV through a Quilt that covered the National Mall. We hope that the book will help catalyze such action and we look forward to your thoughts and suggestions.
Visit the Ending Parkinson’s Disease book website or email your suggestions to Info@EndingPD.org. The book is available at Amazon and Barnes & Noble.
All proceeds will be donated to efforts to end this debilitating disease.
Article written by: Ray Dorsey, MD, and Michael Okun, MD.
Related Blog Posts

20 Parkinson’s-Friendly Gifts

8 Tips for Traveling with Parkinson’s