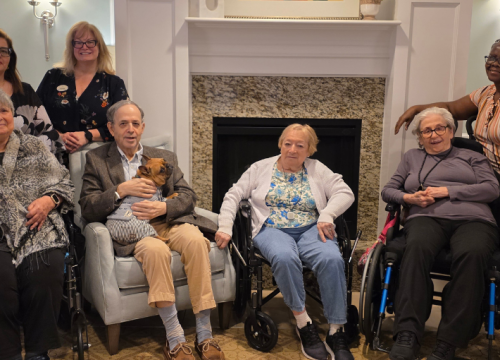Snow Doesn’t Stop Advocates from Making an Impact in Washington
Last week, 300 Parkinson’s disease (PD) advocates from nearly all 50 states convened in Washington, D.C. for the 2018 Parkinson’s Policy Forum. This annual event brings people with PD and their loved ones to our nation’s capital for two days of education and training followed by a day of advocacy action and engagement. Despite a snowstorm that closed Congress’ doors and shut down the streets of Washington, advocates made sure the Parkinson’s community was heard on Capitol Hill.

This year’s Forum was co-sponsored by the Parkinson’s Foundation and The Michael J. Fox Foundation (MJFF). Nine other PD organizations also contributed time and resources to help make the event possible.
“When Parkinson’s groups come together to advocate for change, we amplify our message and have a greater chance of influencing public policy,” says John Lehr, CEO of the Parkinson’s Foundation. “This collaborative approach is key, and we look forward to working together in this way long after the Forum is over.”
The event began with two days of educational panels where advocates learned about recent research advances and current policy issues affecting people with Parkinson’s, their families and care partners. Attendees also received training on how to build relationships with lawmakers and be an effective advocate. Six sessions were streamed live on Facebook and have already garnered nearly 59,000 total views.

Senator Cory Booker, whose late father lived with Parkinson’s, delivered a keynote speech on the morning of Tuesday, March 20. “I tell each and everyone one of you, what you do matters,” he said, underscoring the importance of advocacy. (View the senator’s full remarks.)
On the final day of the event, advocates were scheduled to head to Capitol Hill for congressional meetings but the government shut down due to a snowstorm. Attendees, determined to make their voices heard, rallied and spent the day calling and emailing each of their senators and representatives to ask for increased Parkinson’s research funding in Fiscal Year (FY) 2019. (Congress just passed the FY 2018 spending deal, including a $3 billion boost for the National Institutes of Health, and is now negotiating funding for FY 2019). Advocates also recorded short videos on this topic and posted them to their legislators’ social media feeds. (Search for the hashtag #act4PD on Twitter to see our community in action!)
“I couldn’t see my members of Congress in Washington, but that’s not going to stop me. I know there are many other ways to speak up for the issues that are important to me. I’m already working on setting up meetings with my legislators when they come back to my home state of New Mexico,” said advocate Karen St. Clair. “Advocacy is an activity we must engage in all year round, and I’m going to continue to use what I learned at the Forum to educate my elected officials on what matters to people with PD and their families.”
At the same time Forum attendees were advocating from their hotel, thousands of people across the country joined these efforts by participating in Parkinson’s Advocacy Day. PD community members sent an impressive 14,000 emails to their lawmakers on this day of action, emphasizing the importance of robust federal research funding.
“While the government was closed, some congressional phone lines were open. The staffers who answered our calls told us that they were hearing from many people with PD,” says Ted Thompson, JD, senior vice president of public policy at MJFF. “Our voices are getting through, and Congress is taking note of our needs and priorities. We couldn’t be on Capitol Hill in person, but there’s no doubt in my mind that we made an impact in Washington.”
Related Blog Posts

10 Tips for Playing Pickleball to Stay Active with Parkinson’s