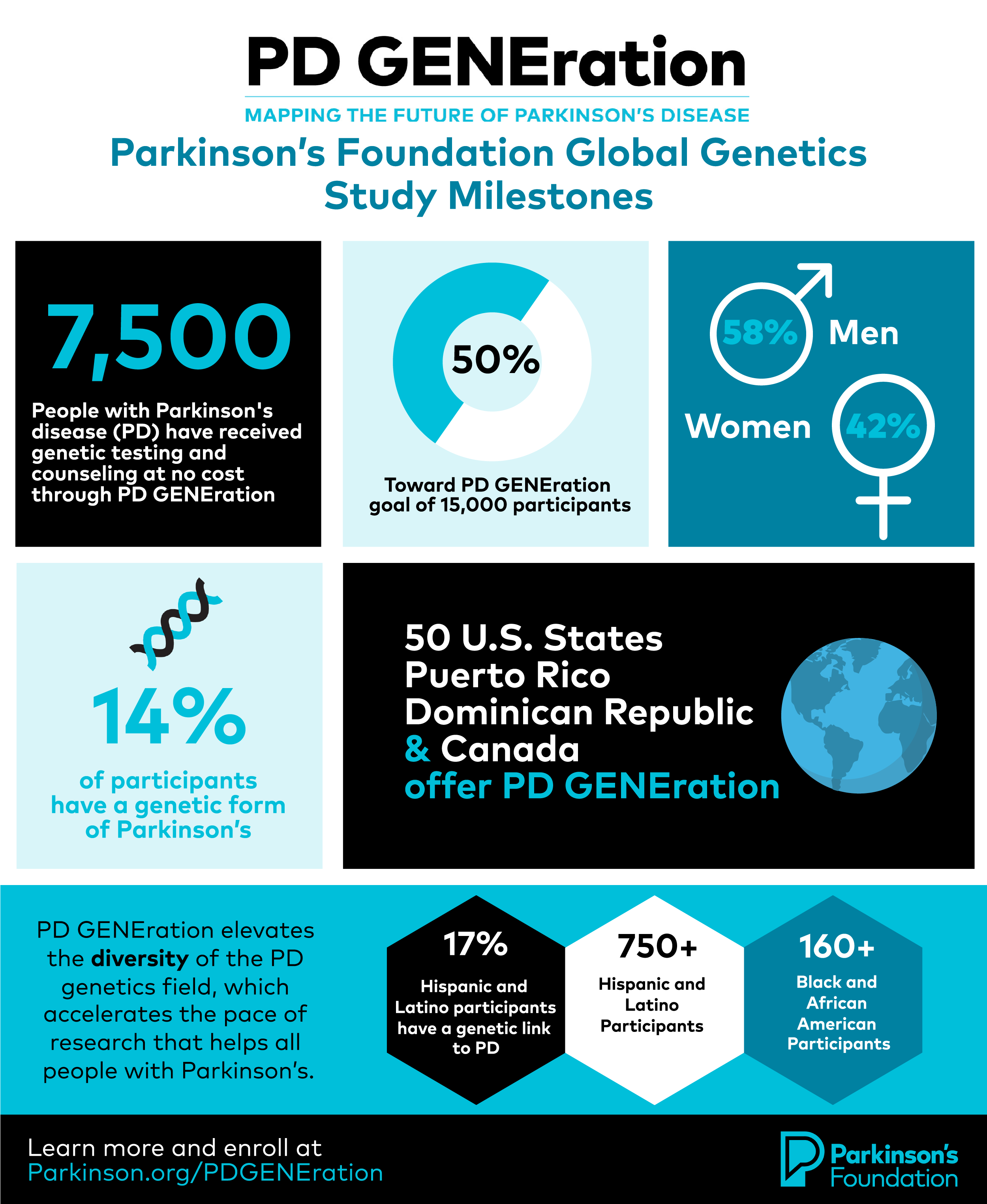
Global Genetics Study Reaches 50% Enrollment Milestone of 7,500 Participants
Genetics is a key step in solving Parkinson’s disease (PD). Understanding how and why Parkinson’s and genetics fit together is a mystery the Parkinson’s Foundation is looking to solve through our landmark study, PD GENEration: Mapping the Future of Parkinson’s Disease. The Parkinson’s Foundation is excited to announce that we are accelerating the path to solving the puzzle of genetics and Parkinson’s through reaching a significant study milestone of providing genetic testing and counseling to 7,500 participants — 12 months ahead of the study recruitment timeline.
“Ensuring that the entire Parkinson’s community — including the 90,000 individuals expected to receive a PD diagnosis this year — has access to their genetic status is as critical as ever,” said James Beck, PhD, chief scientific officer for the Parkinson’s Foundation. “Every PD GENEration participant plays a part not only in their own personal discovery but also in feeding results to researchers which will advance future research.”
Halfway to its goal, the study is on track to provide genetic testing and counseling at no cost to 15,000 people with Parkinson’s, which will establish the largest Parkinson’s genomic dataset in North America.
“I cannot underscore enough how relevant this landmark study is. Our current and rapidly expanding knowledge of this complex disease highly suggests that the likely ‘first cure’ to be discovered will be specific to a genetic mutation for PD, which will then serve as a stepping stone for the ultimate cure for the general PD population,” said Hubert Fernandez, MD, Head of Movement Disorders at Cleveland Clinic and co-chair of the Parkinson Study Group.
In 2022, we expanded the study and made access to genetic testing possible for people with Parkinson’s in all 50 U.S. states, Puerto Rico, the Dominican Republic and Canada. In the next two years, we will introduce PD GENEration to Israel and other countries, bringing a new level of diversity and depth to Parkinson’s genetic research, which can lead to greater insights. So far, 22% of participants represent historically marginalized racial and ethnic groups.
The study continues to expand its reach with the addition of testing sites and collaborations with clinicians in historically excluded communities. This includes a partnership with Morehouse School of Medicine, to make the study more accessible for Black and African American persons in Atlanta, GA. Similarly, the Foundation extensively engages Hispanics and Latinos and provides genetic counseling in English and Spanish, a first of its kind for a study of this scale. Roughly 11% of Latino and Hispanic participants have a 17% genetic link to PD.
“Through the expansion of the PD GENEration study to more populations, we are bringing diversity to genetics data. In turn, this will accelerate the pace of research to help all people with Parkinson’s, regardless of where they live.” — Carlos Singer, MD, professor of neurology at the University of Miami Miller School of Medicine
The study’s data are analyzed in real-time by the Parkinson’s Disease Gene Curation Expert Panel (GCEP), an international working group of genetic experts focused on neurodegenerative diseases formed by the Foundation within the NIH-funded Clinical Genome (ClinGen) Resources. Alongside the Parkinson's Foundation, Roy Alcalay, MD, leads the study as principal investigator.
Currently, investigators have found that 14% of participants have a genetic form of PD — a significant observation compared to the long-standing estimate of 10%.
PD GENEration empowers participants to understand their genetic results. This knowledge can help them make more informed decisions about care and take advantage of clinical studies that are newly accessible to them. Genetics can not only help us uncover potential causes of Parkinson’s, but results from this study can lead to improved treatments and care for everyone with Parkinson’s.
Enroll and help us further PD genetics research at Parkinson.org/PDGENEration.
Related Blog Posts

Navigating the World of Genetic Testing

Neuro Talk: Top Questions About PD GENEration